This Sunday, January 24, the president of Venezuela, Nicolás Maduro, announced to the world that local scientists managed to produce a drug capable of neutralizing the SARS-CoV-2 coronavirus, which causes the COVID-19 disease. The head of State referred to this new finding as «miraculous droplets». It is a novel medicine created and manufactured in Venezuela under the name of «Carvativir».
According to the head of State, it was created with isothymol (isothymol) as the active principle and acts as a selective inhibitor of the main protease of the virus.
“Carvativir, the miraculous droplets of José Gregorio Hernández, neutralizes the symptoms of the coronavirus. From Venezuela to the world!, said Maduro in an address broadcasted on television. In addition, he added that this week it will begin to be mass produced, «so that the National Public Health System has this powerful antiviral».
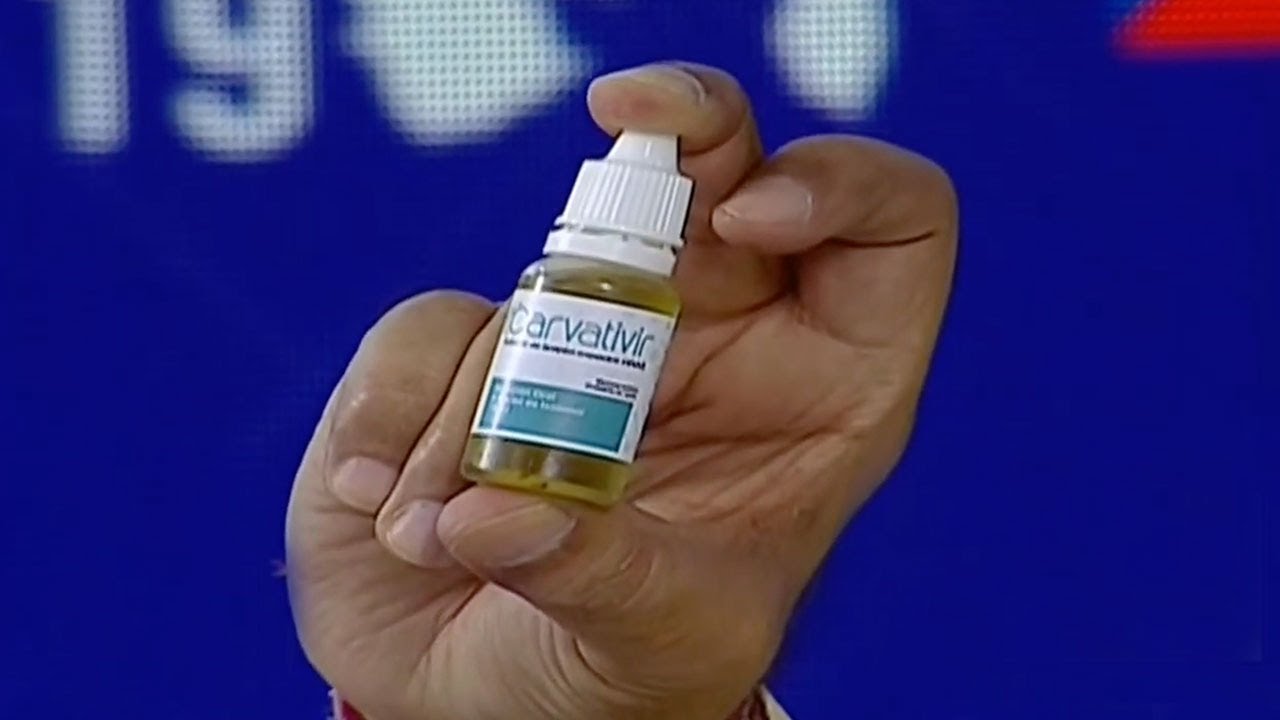
Also, he stressed that this antiviral is «totally harmless», does not generate side effects and has already been tested in trials of which he did not offer details. “It has been used as part of the treatments applied in Venezuela to patients affected with COVID-19, giving good results. Carvativir is a powerful antiviral made in Venezuela. Here we think on how to serve humanity”, he stressed.
The president of Venezuela indicated that casvativir will be incorporated into the «medicine kit» against the coronavirus. He also said that this week he will have a meeting with the health authorities «to establish the system of direct distribution by thousands to the country’s health centers».
Additionally, in his statement he announced that he will coordinate efforts with the countries of the Bolivarian Alliance for the Peoples of Our America (ALBA) to export the medicine.
Carvativir is the result of an investigation funded by the State
The Venezuelan newspaper El Nacional -a media openly opposed to the Maduro government- interviewed “exclusively” Raúl Ojeda Rondón. He is the principal investigator of the project that designed the carvativir.
In the aforementioned interview, the Venezuelan scientist explains that “carvativir reduces hospital recovery time. This is achieved because it accelerates the immune response ”of the patient, something that makes it an effective drug against the coronavirus.
For Rondón “it is important to clarify that it is a line of scientific research of the Venezuelan State through the Ministry of Science and Technology. The studies began in March and ended in December ”of 2020.
Likewise, he explained that, in addition to the Ministry, other organizations are also involved. Among them, he highlighted the Venezuelan Institute of Scientific Research, the Rafael Rangel National Institute of Hygiene, the National Service of Medicine and Forensic Sciences and the Ministry of Health.
Up to now, the investigation has followed three phases, as required by international regulations, monitored and accompanied by the Rafael Rangel National Institute of Hygiene. In addition, a bioethics committee made up of 14 specialists from various areas also participated.
«It is a multicenter, randomized and placebo study», explained the researcher. He pointed out that they used the Kaplan-Meier statistical analysis, used by the University of Oxford and by Russia.
“It is a system that allows us to evaluate the data and establish the different types of behavior of certain clinical parameters with respect to a drug, a molecule and a placebo. (…) This is a scientific investigation, not a political one. Tests were done in Turkey, Iran and the United States», he stressed.
The Medicine: Questions and Answers
What is Carvativir?
It is not a herb or an essential oil. You don’t eat it either. Carvativir is a molecule that is extracted from a phytochemical, purified and modified, and placed in an excipient, which is the vehicle that will transport the molecule in the human body. The excipient we use is squalene, which makes it possible to speed up the immune response of the immune system of the patients we are treating with modified isothymol. We modify it to produce a drug that has therapeutic potential. It is not an oil of oregano.
Carvativir is a purified, synthesized, modified molecule with an excipient that has a high property to stimulate the response of the immune and innate human system. We verified it with clinical and paraclinical tests. With well recognized laboratories.
What steps were taken before the clinical trials?
Before the clinical trials, we had a preclinical stage: several tests were carried out at the IVIC. Carvativir was tested in an in vitro test on infected cells. There, the inhibition of viral replication was verified. In Turkey, a ‘mice model’ was used.
It was verified whether or not there was antiviral activity in a modulator. An in silico trial was subsequently carried out in Venezuela.
What does Carvativir do to coronavirus patients?
Carvativir scientifically presents, with evidence, a therapeutic action. It is used to treat a disease and achieve the recovery of the patient in a certain time. Clinical trials give us an average. Carvativir reduces hospital recovery time by accelerating the immune response. That’s part of the Venezuelan findings.
It was determined that it has antiviral therapeutic activity. The molecule inhibits viral replication in human cells. It decreases the viral load. It also enhances immunomodulatory powers. It decreases – what we call in medicine – the cytokine storm, which is nothing more than the exaggerated response of the immune system, what is known as lung inflammation through atypical pneumonia due to covid-19. That’s where the intubation process comes in.
Have all types of patients been evaluated?
We had patients in mild, moderate and severe condition. There were patients without oxygen, with low-speed oxygen, with high-speed supplemental oxygen, and intubated patients. Carvativir does have therapeutic activity to treat covid-19 because it allows a satisfactory recovery and prevents the cytokine storm and, in many cases, death.

Has the World Health Organization been informed?
Yes. We have been talking with the World Health Organization and the Pan American Health Organization, but now we are going to make it official. We are going to deliver the research we have done in Venezuela with the clinical and paraclinical evidence. We, as researchers, are requesting that the WHO come to validate and certify, what we are doing.
Is the drug miraculous?
It is effective. Recovery is satisfactory. The problem of Covid-19 is immunological. With Carvativir, as I have just explained, it allows a satisfactory response in the recovery of patients. This is a treatment.
With the Carvativir we are reducing recovery times and we are achieving a less aggressive post-covid than what the patients have normally had. That is under evaluation. This is not over.
What comes next?
Phase 4 is coming: mass production of the drug and a pharmacovigilance process. We are going to evaluate how the drug behaves in the general population. We are going to open ourselves to all the scientific communities of the country and abroad so that they can see what we have obtained up to today.